Products


Biosimilars
- NameRemsima®
-
- INNInfliximab
- IndicationsRheumatology, Gastroenterology, Dermatology
- Approved By EMA(EU) Medicines.ie
- NameRemsima® SC
-
- INNInfliximab
- IndicationsRheumatology, Gastroenterology, Dermatology
- Approved By EMA(EU) Medicines.ie
- NameYuflyma®▼
-
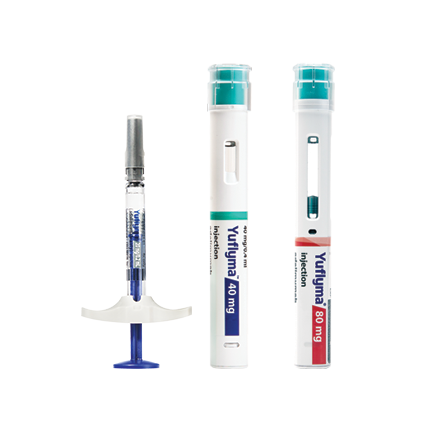
- INNAdalimumab
- IndicationsRheumatology, Gastroenterology, Dermatology
- Approved By EMA(EU) Medicines.ie
- NameSteqeyma®▼
-
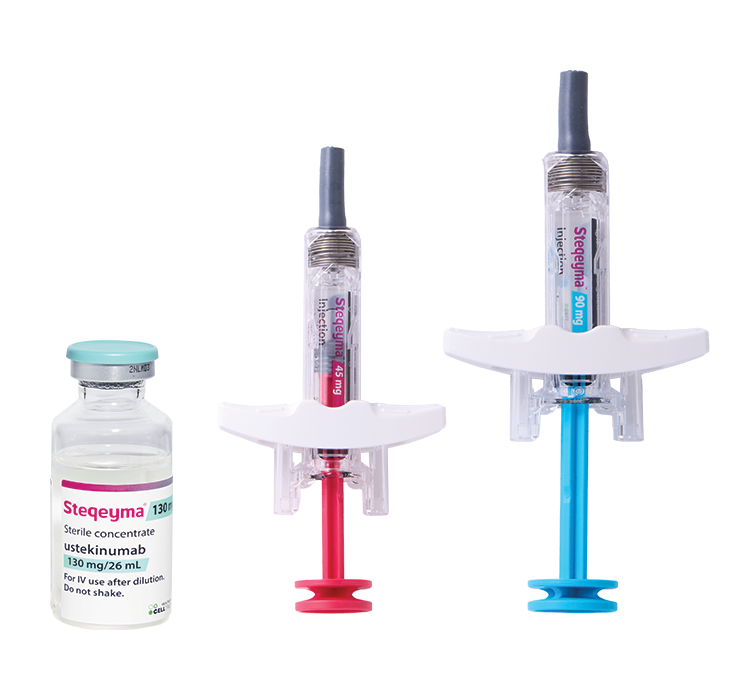
- INNUstekinumab
- IndicationsRheumatology, Gastroenterology, Dermatology
- Approved By EMA(EU) Medicines.ie
This product description is for medical and educational purposes only and not intended to promote the product. For more details, please consult a doctor, nurse or pharmacist.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly to HPRA Pharmacovigilance via www.hpra.ie. By reporting side effects you can help provide more information on the safety of this medicine.
INN, international non-proprietary name
Novel Therapeutics
This product description is for medical and educational purposes only and not intended to promote the product. For more details, please consult a doctor, nurse or pharmacist.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly to HPRA Pharmacovigilance via www.hpra.ie. By reporting side effects you can help provide more information on the safety of this medicine.
INN, international non-proprietary name